RJH Biosciences Inc.
Products Orverview
Product Features
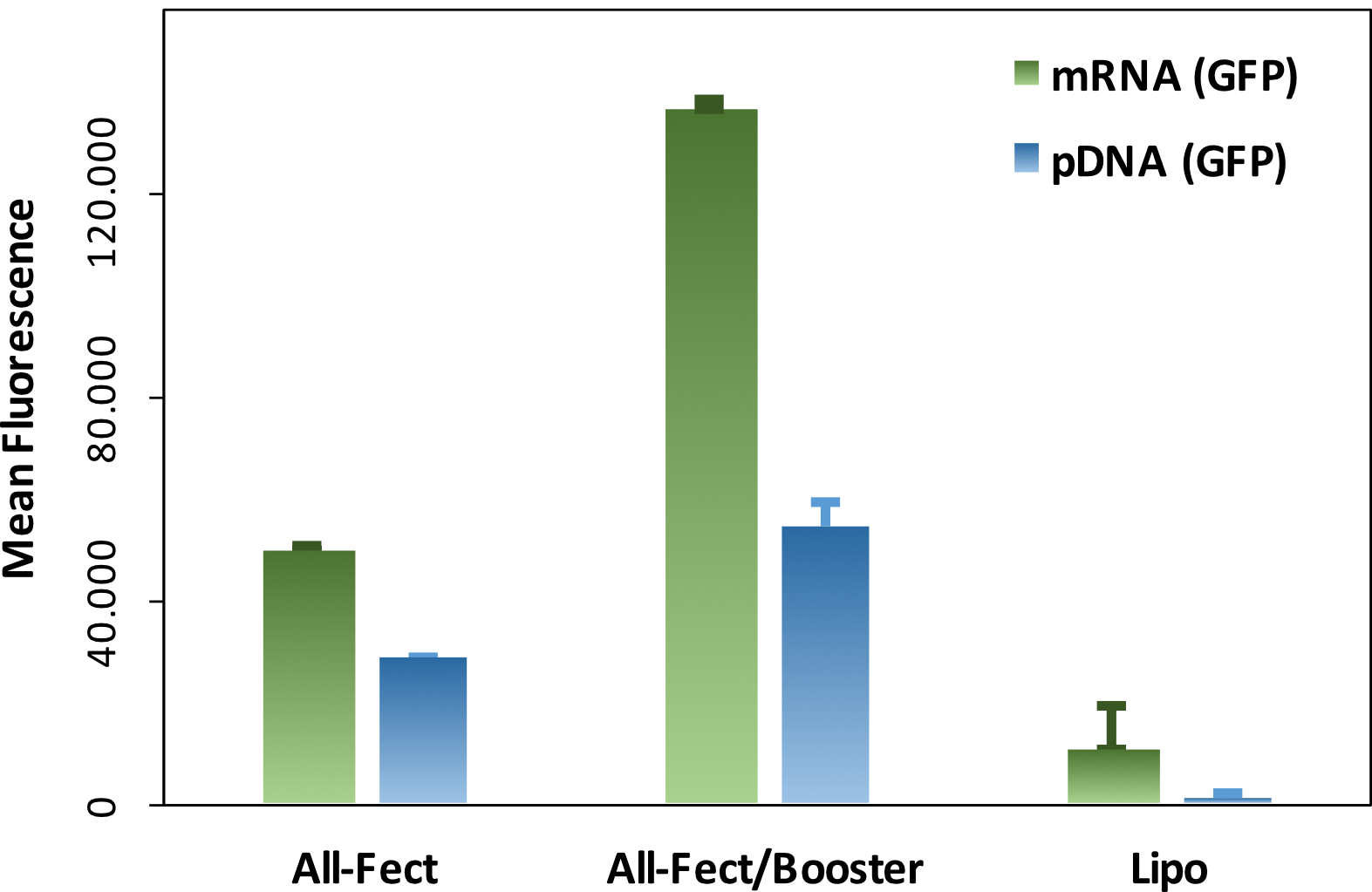
All-Fect
Designed as a siRNA pDNA transfection reagent it can be used for either siRNA knockdown, plasmid DNA transformation or siRNA pDNA co-delivery. It is particularly suitable for mesenchymal stem cells from cord blood and bone marrow, and highly differentiated cells such as smooth muscle cells, and endothelial cells.
Download Information: All-Fect Brochure
Benefits of the reagent:
- High transfection efficiency
Provides 2 to 3-fold higher efficacy in the presences of serum
- Simple Protocol
No need to change tissue culture medium during transfection
- Lower Toxicity
Less toxic compared to commercial transfection reagents, leading to better retention of normal cellular physiology
References:
- Hsu and Uludağ. Biomaterials (2012) 33: 7834-7848.
- Remant Bahadur et al., J. Materials Chemistry B (2015) 3: 3972-3982.
- Wang et al., J. Surgical Research (2013) 183: 8-17.

Feature of Products
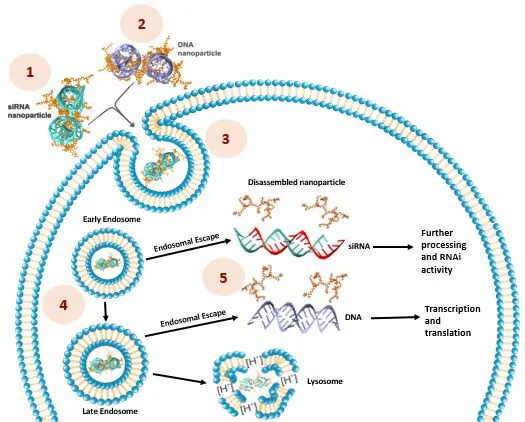
RJH developed broadly acting transfection reagents to modify mammalian cells with plasmid DNA, siRNA, mRNA and other nucleic acids. The foundation of our transfection reagents is based on cationic lipopolymers with optimal balance of cationic charge and hydrophobic (lipid) group. By systematically altering the polymeric backbone and the nature of lipid group, a library of transfection reagents has been generated. Some of the transfection reagents are broadly acting, functioning in different cell types with different nucleic acids. Others display high specificity, where they are exceptionally effective in particular cell types for particular nucleic acid delivery.
The key advantages of our delivery vehicles are:
- Multivalent interactions with nucleic acids leading to strong binding of cargo that withstand disruptive forces in transit through cell membranes.
- Synergistic effects due to cationic and lipidic binding that coat the cargo and protect it from nucleases.
- Lipidic moieties that enhance interactions with cell memblane and internalization.
- pH buffering capacity that facilitate escape of cargo from endosomes.
- Tailored formulations to free nucleic acids once internalized in cytoplasm.
The transfection reagents have been optimized for the following cell models and applications. For a classification our transfection reagents by cell type, please see Selection Guide.
| Primary Cells | |
|---|---|
|
Attachment Dependent
|
Attachment Independent
|
| Cell Lines | |
|
Attachment Dependent
|
Attachment Independent
|
| Animal Models | |
|
|
Background
RJH’s R&D Focus
One area of focus for RJH Biosciences is to implement RNA interference (RNAi) via delivery of short interfering RNA (siRNA). Our initial therapeutic application is blood cancers, while recognizing that the RNAi activity can be implemented in the treatment of a large range of human cancers and other diseases. Another focus is direct administration of plasmid DNA (pDNA) to express therapeutic proteins in situ, with applications in immunotherapy.
Why study blood cancers and immunotherapies with nucleic acid therapeutics?
There are three types of blood cancers: Leukemia, lymphoma, and myeloma. Leukemia is characterized by highly proliferating, abnormal white blood cells [1]. Lymphoma and myeloma are respectively cancers of the lymphatic system and plasma cells which greatly effect the immune system [2,3]. These three cancers are difficult to treat and the current treatments are limited in efficacy, especially at the end stage of the disease.
The use of nucleic acid-based therapeutics can eradicate these cancers in two primary ways, with RNAi technology and cell-based immunotherapy. The use of RNAi is being increasingly explored in the treatment of the blood cancers. Polynucleotides such as siRNA has aided the downregulation of oncogenes and can be designed to support specific abnormalities in individual patients, making it a ‘personal’ strategy with a universal technological design [4]. Due to siRNA’s potential in blood cancer therapies, we are currently focusing on siRNA therapeutics in our R&D projects, targeting disease-driving oncogenes and inducing apoptosis in the malignant cells.
Another strategy that is being explored for treating blood cancers is the use of immunotherapy. Immunotherapeutic strategies include the use of antibodies, stem cell transplants, cytokines, small molecules among others [5]. However, a more recent approach is genetic therapy by using engineered cells, also known as Cell Transfer Therapy. This method works by taking patients’ own immune cells such as but not limited to T-cells, B-cells, and NK cells. The immune cells genome is engineered to support various therapeutic strategies that may involve neoantigen expression and presentation on immune cell surface, and then are reintroduced into the host [5]. The modified cells are ultimately designed to target and remove the malignant cells. This strategy is highly advantageous as T-cells can ‘seek’ and destroy the malignant cells in the blood system. While this approach has been promising in blood cancers, it can be also used in other solid cancers. As the foundation of immunotherapy relies on nucleic acid introduction into patient cells, efficient delivery of nucleic acids is imperative for success. Our transfection reagents offer the best in class vehicles to undertake such a delivery.
Nanomedicine based on nucleic acid therapeutics is a large component to personalized cancer therapies and immunotherapies. The RJH Biosciences strives to provide quality transfection reagents, whether it involves the delivery of our own nucleic acid candidates or our customers’.
Woods, N. et al. (2006) Therapueti gene causing lymphoma. Nature. 440, 1123.
Mahindra, A. et al. (2012) Latest advances and current challenges in the treatment of multiple myeloma. Nature Reviews Clinical Oncology. 9, 135-143.
Uludağ, H. et al. (2016) Current attempts to implement siRNA-based RNAi in leukemia models. Drug Discovery Today. 21, 1412-1420.
Zou, W. (2006) Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology. 6, 295-307.
Product List
All-Fect
pDNA, siRNA and co-delivery reagents for a broad range of cells
| Catalog No. | Product Name | Volume | Concentration | Trans-Booster |
|---|---|---|---|---|
| 10-10 | All-Fect | 0.75 mL | 1 mg/mL | – |
| 10-20 | All-Fect | 1.5 mL | 1 mg/mL | – |
| 10-40 | All-Fect Kit | 0.75 mL | 1 mg/mL | 0.75 mL at 0.4 mg/mL |
| 10-50 | All-Fect Kit | 1.5 mL | 1 mg/mL | 1.5 mL at 0.4 mg/mL |
| 10-60 | ALL-Fect In Vivo Kit | 1 mL | 5 mg/mL | 1 mL at 2 mg/mL |
Product Page of RJH: siRNA, microRNA and ASO Delivery
Prime-Fect
Reagent of choice for tough to transfect primary and stem cells
| Catalog No. | Product Name | Volume | Concentration | Trans-Booster |
|---|---|---|---|---|
| 20-10 | Prime-Fect | 0.75 mL | 1 mg/mL | – |
| 20-20 | Prime-Fect | 1.5 mL | 1 mg/mL | – |
| 20-40 | Prime-Fect Kit | 0.75 mL | 1 mg/mL | 0.75 mL at 0.4 mg/mL |
| 20-50 | Prime-Fect Kit | 1.5 mL | 1 mg/mL | 1.5 mL at 0.4 mg/mL |
Product Page of RJH: Prime-Fect: Primary Cell Transfection Reagent
Leu-Fect A & B
Specialized reagents leukemia and suspension cells
| Catalog No. | Product Name | Volume | Concentration | Trans-Booster |
|---|---|---|---|---|
| 30-10 | Leu-Fect A | 0.75 mL | 1 mg/mL | – |
| 30-20 | Leu-Fect A | 1.5 mL | 1 mg/mL | – |
| 40-10 | Leu-Fect B | 0.75 mL | 1 mg/mL | – |
| 40-20 | Leu-Fect B | 1.5 mL | 1 mg/mL | – |
Product Page of RJH: Leu-Fect A & B: Leukaemia Cell Transfection Reagents
mRNA-Fect
Highly effective transfection reagent optimized for mRNA delivery
| Catalog No. | Product Name | Volume | Concentration | Trans-Booster |
|---|---|---|---|---|
| 80-10 | mRNA-Fect | 0.75 mL | 1 mg/mL | – |
| 80-20 | mRNA-Fect | 1.5 mL | 1 mg/mL | – |
| 80-30 | mRNA-Fect In Vivo | 1 mL | 5 mg/mL | – |
| 80-40 | mRNA-Fect Kit | 0.75 mL | 1 mg/mL | 0.75 mL at 0.4 mg/mL |
| 80-50 | mRNA-Fect Kit | 1.5 mL | 1 mg/mL | 1.5 mL at 0.4 mg/mL |
| 80-60 | mRNA-Fect In Vivo Kit | 1 mL | 5 mg/mL | 1 mL at 2 mg/mL |
Product Page of RJH: mRNA Transfection Reagents
CRISP-Fect
Highly effective transfection reagent optimized for ribonucleoprotein (RNP) delivery to both attachment-dependent and suspension-growing cells.
| Catalog No. | Product Name | Volume | Concentration | Trans-Booster |
|---|---|---|---|---|
| 90-10 | CRISP-Fect | 0.75 mL | 1 mg/mL | 0.75 mL at 0.4 mg/mL |
| 90-20 | CRISP-Fect | 1.5 mL | 1 mg/mL | 1.5 mL at 0.4 mg/mL |
Product Page of RJH: CRISPR Transfection Reagents
Transfection Reagent Selection Guide
The reagents were tested in culture by using plasmid DNA (pDNA), short interfering RNA (siRNA), messenger RNA (mRNA), microRNA (miR), antisense oligonucleotide (ASO) or Cas9/sgRNA ribonucleoprotein (RNP) complex.
Recommended Transfection Reagents in Different Cell Types
| Cell Type | All-Fect | Leu-Fect-A | Leu-Fect-B | Prime-Fect | mRNA-Fect | CRISP-Fect | |
|---|---|---|---|---|---|---|---|
|
Primary Cells
|
Umbilical Cord Blood Derived Mesenchymal Stem Cells (UCB-MSC) | pDNA | pDNA | mRNA | |||
| Bone Marrow Derived Mesenchymal Stem Cells (BM-MSC) | pDNA | pDNA | mRNA | ||||
| Vascular smooth muscle Cells (VSMCs) | pDNA | pDNA | mRNA | ||||
| Human Umbilical Vein Endothelial Cells (HUVECs) | pDNA | mRNA | |||||
| Mononuclear Cells from Healthy Individuals and Leukemia (CML, AML, ALL) Patients | siRNA | siRNA | mRNA | ||||
| Human Foreskin Fibroblast Cells | pDNA | pDNA | mRNA | ||||
| Rat Primary Sympathetic Neurons | pDNA | mRNA | |||||
|
Cell Lines
|
Kidney Fibroblast Cells (293-T) | pDNA | pDNA | ||||
| Kidney Epithelial Cells (MDCK) | siRNA | ||||||
| Breast Cancer/Melanoma Cells (MDA-MB-436) | pDNA, siRNA | siRNA | mRNA | RNP | |||
| Breast Cancer Cells (MDA-MB-231, MDA-MB-468, Sum-149PT, MCF-7) | pDNA, siRNA | siRNA | mRNA | RNP | |||
| Human Lymphoma Cells (U-937) | pDNA | pDNA | |||||
| Chronic Myeloid Leukaemia Cells (K562) | siRNA, miR | mRNA | |||||
| Acute Myeloid Leukemia Cells (KG1, KG1A, THP-1, MV4-11, MOLM-13) | siRNA | mRNA | |||||
| Acute Lymphocytic Leukemia (RS;4-11) | siRNA | ||||||
| Human Lung Cancer Cells (A549, Calu-3, H1975) | pDNA, siRNA | siRNA | mRNA | ||||
| Human Colon Cancer (HCT-116) | pDNA | siRNA | mRNA | ||||
| Human Myoblasts | ASO | ||||||
| Jurkat Cells | pDNA | mRNA | |||||
| Neuronal Cell Line N2A-97Q | pDNA | siRNA | pDNA | mRNA |
Recommended Reagents for Use in Animal Models
| Configuration | All-Fect | Leu-Fect-A | Leu-Fect-B | mRNA-Fect |
|---|---|---|---|---|
| Local Injection into Host Tissue | pDNA | siRNA | siRNA | mRNA |
| Systemic Injection (IV, SC, IP) | pDNA | siRNA | siRNA | mRNA |
| Local Graft (optimal reagent for parent cells) | pDNA | siRNA | siRNA | mRNA |
| Systemic Graft (optimal reagent for parent cells) | pDNA | siRNA | siRNA | mRNA |
| Implantation with a Matrix | pDNA | mRNA |
The reagents are tested in indicated configurations in rodent models. Graft models are based on human cells grown in mice (xenografts) either locally (subcutaneously) or systemicly after IV injection of cells.
Resources
Example of Use
Feedback from independent researchers
Implementing CRISPR-Cas9 Technology using Transfection Reagents from RJH Biosciences
Killing Lung Cancer A549 Cells with RJH Reagents and Cytotoxic siRNAs
Transfecting Colon Cancer HCT-116 Cells with RJH Reagents to silence Polynucleotide Kinase 3′-Phosphatase (PNKP) Expression
Use of RJH Transfection Reagents in Antisense Oligonucleotide Delivery
Reagents for pDNA and siRNA delivery for TNBC cells
Application Notes
Reagents for Antisense Oligonucleotide (ASO) Delivery
Comparing Lipofection to RJH Reagents
mRNA Modification of PBMCs
mRNA-Fect Transfection Reagent to Deliver mRNA in Breast Cancer Cells
Transfecting Triple-Negative Breast Cancer MDA-MB-231 Cells with Plasmid DNA and siRNA by using ALL-Fect and Prime-Fect
Use of RJH Transfection Reagents in Co-Delivery of Plasmid DNA and short interfering RNA
RJH Transfection Reagents in siRNA Library Screens
microRNA Delivery to Leukemic Cells
Q&A
All-Fect Transfection Protocols
Prime-Fect Transfection Protocols
Leu-Fect A Transfection Protocols
Leu-Fect B Transfection Protocols
Trans-Booster Transfection Protocols
In Vivo DNA-Fect Transfection Protocols
Please contact us for any inquiries, questions, or information requests.
Tokyo Future Style, Inc.
info@tokyofuturestyle.com
TEL:029-851-9222 FAX:029-851-9220