ACROBioSystems – GMP Grade Cytokines

Intoduction
Immune cell therapy, represented by CAR-T/NK cells has shown significant therapeutic effects in the treatment of various malignant tumors such as leukemia, lymphoma, and multiple myeloma. As more and more immune cell therapies enter the stage of clinical research, quality management systems have attracted more attention from the industry. During the process of immune cell therapy products, cytokines such as IL-15, IL-7, IL-21 are used as a key raw material for T/NK cell activation and amplification. Therefore, safe, effective, and compliant cytokines are crucial for the success of R&D processes and applications of immune cell therapy drugs.
ACROBiosystems is committed to the development of high-quality reagents that are used in the clinical stage of immune cell therapy drugs. Based on the GMP-grade quality management system platform, combined with the production specifications of cell therapy drugs, ACROBiosystems have successfully developed a series of high-quality GMP-grade cytokines such as IL-15, IL-7, IL-21. These products are produced with strict quality management and drug-level release testing standards. ACROBiosystems’s GMP-grade cytokines* can better assist the clinical research of immune cell therapy drugs and accelerate the global regulatory approval of biological products.
* ACROBiosystems GMP grade products are designed for research, manufacturing use, or ex vivo use. CAUTION: Not intended for human in vivo applications.
GMP QMS
Quality Management System
- Manufactured and QC tested under GMP compliance
- Designed under ISO 9001:2015 and ISO 13485:2016
- Animal-Free materials
- Materials purchased from approved suppliers
- ISO 5 cleanrooms and automatic filling equipment
- Qualified and well-trained personnel
- Quality-related documents reviewed and approved by QA
- Fully batch production and control records
- Equipment maintenance and calibration
- Validation of analytical procedures
- Stability studies conducted
- Comprehensive regulatory support files


Strict quality standards (Example for GMP IL-15 release standard)
- SDS-PAGE>95%
- Endotoxin level less than 10 EU/mg
- Residual Host Cell DNA content less than 0.02ng/μg
- Residual Host Cell Protein content less than 0.5ng/ug
- Biological activity >0.8 x 107 IU/mg (Reference the WHO Human IL-15 (NIBSC code: 90/530) as standard)
- Microbial testing
- Mycoplasma testing
- In vitro virus assay
- Batch-to-batch consistency
- Comprehensive stability data support (accelerated, freeze-thaw, long-term, shipping stability verification)
Product Features
Strict Quality Control Standards
- 16 quality control standards.
- Excellent safety profile (testing for sterility, mycoplasma, endotoxin, and residual impurities).
- High stability and batch-to-batch consistency.
GMP Grade Quality Management System
- ISO 5 cleanrooms used for filling.
- Raw materials and packing materials are registered.
- Facilities are available for online and on-site audits.
Accelerating Global Regulatory Approval of Biological Products
- A comprehensive set of regulatory documents is available.
- Validation reports for analysis methods are available by request.
- FDA DMF filed
Product List
| GMP Grade Products | Residue Detection Kits | ||
|---|---|---|---|
| Cat. No. | Product Name | Cat. No. | Product Name |
| GMP-CA9S18 | GMP GENPower™ NLS-Cas9 Nuclease | – | This residue detection kit is still under development. |
| GMP-DL4H23 | GMP Biotinylated Human DLL4 Protein, His,Avitag™ | – | This residue detection kit is still under development. |
| GMP-DL4H28 | GMP Human DLL4 Protein, Fc Tag | – | This residue detection kit is still under development. |
| GMP-FGCH17 | GMP Human FGF basic Protein | – | This residue detection kit is still under development. |
| GMP-FLLH28 | GMP Human Flt-3 Ligand Protein | – | This residue detection kit is still under development. |
| GMP-IFGH24 | GMP Human IFN-gamma Protein | – | This residue detection kit is still under development. |
| GMP-ILBH16 | GMP Human IL-1 beta Protein | – | This residue detection kit is still under development. |
| GMP-L02H14 | GMP Human IL-2 Protein | CRS-A003 | resDetect™ Human Interleukin-2 (IL-2) ELISA Kit (Residue Testing) |
| GMP-L04H26 | GMP Human IL-4 Protein | CRS-A004 | resDetect™ Human Interleukin-4 (IL-4) ELISA Kit (Residue Testing) |
| GMP-L06H27 | GMP Human IL-6 Protein | CRS-A005 | resDetect™ Human Interleukin-6 (IL-6) ELISA Kit (Residue Testing) |
| GMP-L07H24 | GMP Human IL-7 Protein | CRS-A025 | resDetect™ Human Interleukin-7 (IL-7) ELISA Kit (Residue Testing) |
| GMP-L15H13 | GMP Human IL-15 Protein | CRS-A024 | resDetect™ Human Interleukin-15 (IL-15) ELISA Kit (Residue Testing) |
| GMP-L21H25 | GMP Human IL-21 Protein | CRS-A010 | resDetect™ Human Interleukin-21 (IL-21) ELISA Kit (Residue Testing) |
| GMP-MBS001 | GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads | – | This residue detection kit is still under development. |
| GMP-MC0323 | GMP Monoclonal Anti-Human CD3 Antibody (OKT3) | CRS-A015 | resDetect™ Anti-CD3 Antibody ELISA Kit |
| GMP-MC2824 | GMP Monoclonal Anti-Human CD28 Antibody | CRS-A014 | resDetect™ Anti-CD28 Antibody ELISA Kit |
| GMP-NUES19 | GMP GENIUS™Nuclease | CRS-A016 | resDetect™ GENIUS™ Nuclease ELISA Kit (Residue Testing) |
| GMP-SCFH25 | GMP Human SCF Protein | – | This residue detection kit is still under development. |
| GMP-TNAH23 | GMP Human TNF-alpha Protein | – | This residue detection kit is still under development. |
| GMP-VC1H25 | GMP Human VCAM-1 Protein, Fc Tag | – | This residue detection kit is still under development. |
| GMP-VE5H23 | GMP Human VEGF165 Protein | – | This residue detection kit is still under development. |
Related Page: Full list of proteins for cell culture Tokyo Future Style offers
Data
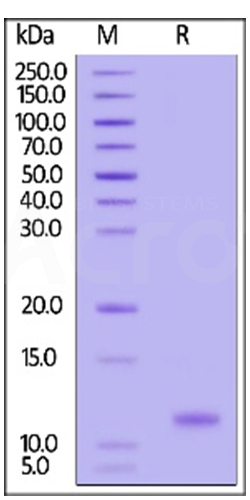
High purity
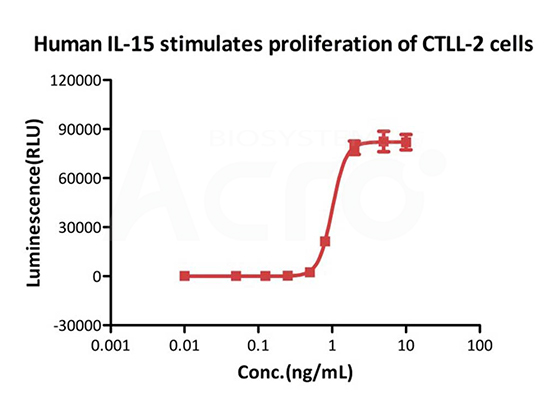
High bioactivity
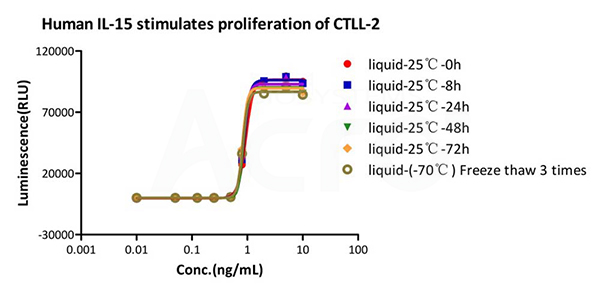
High stability
Validation of Accelerate and Freeze-thaw stability
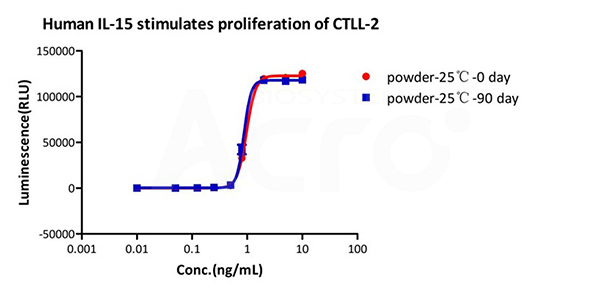
Long-term stability testing (25℃)
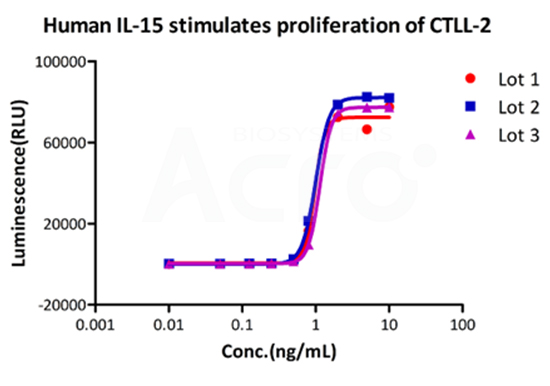
High batch-to-batch consistency
References
- [1] Libby, Kendra A, and Xiaolei Su. Imaging Chimeric Antigen Receptor (CAR) Activation[J]. Methods in molecular biology. 2020, 153-160.
- [2] Chinese Pharmacopoeia
- [3] Corrected Draft Guidance for Industry: Chemistry, Manufacturing,and Control(CMC) Information for Human Gene Therapy Investigation New Durg Applications(INDs). FDA, CBER, 2018.7.20
Please contact us for any inquiries, questions, or information requests.
Tokyo Future Style, Inc.
info@tokyofuturestyle.com
TEL:029-851-9222 FAX:029-851-9220

